April 3, 2025
Definitions Used for Off-the-Shelf versus Custom Fitted Prefabricated Orthotics (Braces) – Correct Coding – Revised
Joint DME MAC Publication
As part of the 2014 and 2015 HCPCS update, codes were created describing certain off-the-shelf (OTS) orthotics. Some of these codes parallel codes for custom fitted versions of the same items. Refer to the table at the end of this article for a listing of codes.
When providing these items suppliers must:
- Provide the product that is specified by the treating practitioner
- Be sure that the treating practitioner's medical record justifies the need for the type of product (i.e., Prefabricated versus Custom Fabricated)
- Only bill for the HCPCS code that accurately reflects both the type of orthosis and the appropriate level of fitting.
- Have detailed documentation in the supplier's record that justifies the code selected
The following definitions will be used for correct coding of these items.
Off-the-shelf (OTS) orthotics are:
- Items that are prefabricated.
- They may or may not be supplied as a kit that requires some assembly. Assembly of the item and/or installation of add-on components and/or the use of some basic materials in preparation of the item does not change classification from OTS to custom fitted.
- OTS items require minimal self-adjustment for fitting at the time of delivery for appropriate use and do not require expertise in trimming, bending, molding, assembling, or customizing to fit an individual.
- This fitting does not require expertise of a certified orthotist or an individual who has specialized training in the provision of orthoses to fit the item to the individual beneficiary.
The term "minimal self-adjustment" is defined at 42 CFR §414.402 as an adjustment the beneficiary, caretaker for the beneficiary, or supplier of the device can perform and that does not require the services of a certified orthotist (that is, an individual who is certified by the American Board for Certification in Orthotics and Prosthetics, Inc., or by the Board for Orthotist/Prosthetist Certification) or an individual who has specialized training. For example, adjustment of straps and closures, bending or trimming for final fit or comfort (not all-inclusive) fall into this category.
As a reminder, use of CAD/CAM or additive manufacturing techniques alone does not designate a product as custom fabricated.
Custom fitted orthotics are:
- Devices that are prefabricated.
- They may or may not be supplied as a kit that requires some assembly. Assembly of the item and/or installation of add-on components and/or the use of some basic materials in preparation of the item does not change classification from OTS to custom fitted.
- Classification as custom fitted requires more than minimal self-adjustment at the time of delivery in order to provide an individualized fit, i.e., the item must be trimmed, bent, molded (with or without heat), or otherwise modified resulting in alterations beyond minimal self-adjustment.
- This fitting at delivery does require expertise of a certified orthotist or an individual who has specialized training in the provision of orthosis to fit the item to the individual beneficiary.
"More than minimal self-adjustment" is defined as changes made to achieve an individualized fit during the final fitting at the time of delivery of the item that requires the expertise of a certified orthotist or an individual who has specialized training in the provision of orthotics in compliance with all applicable Federal and State licensure and regulatory requirements. A certified orthotist is defined as an individual who is certified by the American Board for Certification in Orthotics and Prosthetics, Inc., or by the Board for Orthotist/Prosthetist Certification.
As a reminder, use of CAD/CAM or additive manufacturing techniques alone does not designate a product as custom fabricated.
Kits are:
- A collection of components, materials and parts that require further assembly before delivery of the final product.
- The elements of a kit may be packaged and complete from a single source or may be an assemblage of separate components from multiple sources by the supplier.
A summary classification algorithm and table is included at the end of this document to assist with determinations about the type of product and correct code selection.
Refer to the Contractor Supplier Manual, applicable Local Coverage Determinations and related Policy Articles for additional information about other coverage, coding and documentation requirements.
For questions about correct coding, contact the Pricing, Data Analysis and Coding (PDAC) HCPCS Helpline at (877) 735-1326 during the hours of 9:30 a.m. to 5:00 p.m. ET, Monday through Friday. You may also visit the PDAC website![]() to chat with a representative or select the Contact Us
to chat with a representative or select the Contact Us![]() button at the top of the PDAC website for email, FAX, or postal mail information.
button at the top of the PDAC website for email, FAX, or postal mail information.
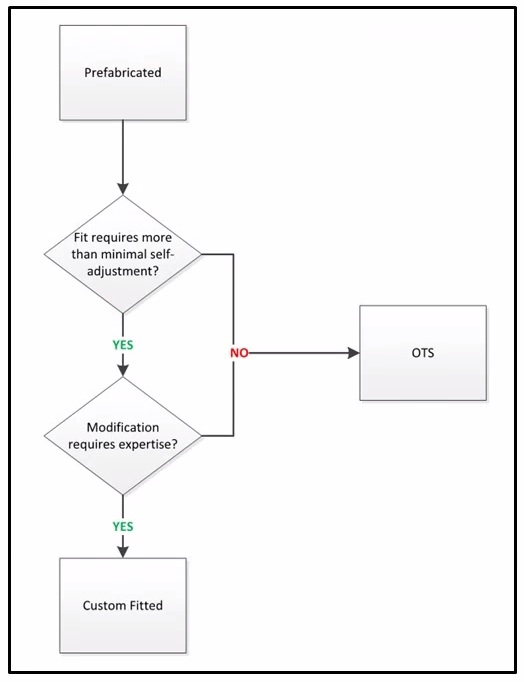
Classification Algorithm – Overview of Criteria
Determining Proper Coding of Prefabricated Orthotics
The following question and answer relate to whether a prefabricated orthotic is properly billed using a code for a custom fitted orthotic versus one furnished off-the-shelf and does not address medical necessity for the item. The descriptors for the HCPCS codes for custom fitted orthotics include the following nomenclature:
- Off-the-shelf (OTS) – Prefabricated item that requires minimal self-adjustment e.g., being trimmed, bent, molded, assembled, or otherwise adjusted to fit the beneficiary. Minimal self-adjustment does not require the expertise of a certified orthotist or an individual with specialized training in the provision of orthotics.
- Custom fitted – Prefabricated item that requires more than minimal self-adjustments e.g., has been trimmed, bent, molded, assembled, or otherwise customized to fit a specific patient by a certified orthotist or an individual with specialized training in the provision of orthotics.
Question: Is the prefabricated orthotic furnished with custom fitting that is and can only be provided by an individual with expertise or furnished off-the-shelf (OTS)?
Answer: Classification depends on (1) what must be done at final fitting and (2) who must do it. Expertise of a qualified practitioner and more than minimal self-adjustment at the time of delivery qualify the items for classification as custom fitted. Failure to meet either criterion 1 or 2, the item would be classified as off-the-shelf.
How to Decide What Code Type for Prefabricated Orthotic
HCPCS Code Table
Note: Not all codes listed have a corresponding medical policy. There are policies for ankle-foot and knee-ankle-foot orthoses, knee orthoses, and spinal orthoses. There are no medical policies for hip, wrist, hand, finger, elbow, or shoulder orthoses.
| HCPCS | Custom Fitted Codes | HCPCS | Off-the-Shelf Codes |
|---|---|---|---|
| L0454 | TLSO FLEXIBLE, PROVIDES TRUNK SUPPORT, EXTENDS FROM SACROCOCCYGEAL JUNCTION TO ABOVE T-9 VERTEBRA, RESTRICTS GROSS TRUNK MOTION IN THE SAGITTAL PLANE, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISKS WITH RIGID STAYS OR PANEL(S), INCLUDES SHOULDER STRAPS AND CLOSURES, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0455 | TLSO, FLEXIBLE, PROVIDES TRUNK SUPPORT, EXTENDS FROM SACROCOCCYGEAL JUNCTION TO ABOVE T-9 VERTEBRA, RESTRICTS GROSS TRUNK MOTION IN THE SAGITTAL PLANE, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISKS WITH RIGID STAYS OR PANEL(S), INCLUDES SHOULDER STRAPS AND CLOSURES, PREFABRICATED, OFF-THE-SHELF |
| L0456 | TLSO, FLEXIBLE, PROVIDES TRUNK SUPPORT, THORACIC REGION, RIGID POSTERIOR PANEL AND SOFT ANTERIOR APRON, EXTENDS FROM THE SACROCOCCYGEAL JUNCTION AND TERMINATES JUST INFERIOR TO THE SCAPULAR SPINE, RESTRICTS GROSS TRUNK MOTION IN THE SAGITTAL PLANE, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISKS, INCLUDES STRAPS AND CLOSURES, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0457 | TLSO, FLEXIBLE, PROVIDES TRUNK SUPPORT, THORACIC REGION, RIGID POSTERIOR PANEL AND SOFT ANTERIOR APRON, EXTENDS FROM THE SACROCOCCYGEAL JUNCTION AND TERMINATES JUST INFERIOR TO THE SCAPULAR SPINE, RESTRICTS GROSS TRUNK MOTION IN THE SAGITTAL PLANE, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISKS, INCLUDES STRAPS AND CLOSURES, PREFABRICATED, OFF-THE-SHELF |
| L0466 | TLSO, SAGITTAL CONTROL, RIGID POSTERIOR FRAME AND FLEXIBLE SOFT ANTERIOR APRON WITH STRAPS, CLOSURES AND PADDING, RESTRICTS GROSS TRUNK MOTION IN SAGITTAL PLANE, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON INTERVERTEBRAL DISKS, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0467 | TLSO, SAGITTAL CONTROL, RIGID POSTERIOR FRAME AND FLEXIBLE SOFT ANTERIOR APRON WITH STRAPS, CLOSURES AND PADDING, RESTRICTS GROSS TRUNK MOTION IN SAGITTAL PLANE, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON INTERVERTEBRAL DISKS, PREFABRICATED, OFF-THE-SHELF |
| L0468 | TLSO, SAGITTAL-CORONAL CONTROL, RIGID POSTERIOR FRAME AND FLEXIBLE SOFT ANTERIOR APRON WITH STRAPS, CLOSURES AND PADDING, EXTENDS FROM SACROCOCCYGEAL JUNCTION OVER SCAPULAE, LATERAL STRENGTH PROVIDED BY PELVIC, THORACIC, AND LATERAL FRAME PIECES, RESTRICTS GROSS TRUNK MOTION IN SAGITTAL, AND CORONAL PLANES, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON INTERVERTEBRAL DISKS, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0469 | TLSO, SAGITTAL-CORONAL CONTROL, RIGID POSTERIOR FRAME AND FLEXIBLE SOFT ANTERIOR APRON WITH STRAPS, CLOSURES AND PADDING, EXTENDS FROM SACROCOCCYGEAL JUNCTION OVER SCAPULAE, LATERAL STRENGTH PROVIDED BY PELVIC, THORACIC, AND LATERAL FRAME PIECES, RESTRICTS GROSS TRUNK MOTION IN SAGITTAL, AND CORONAL PLANES, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON INTERVERTEBRAL DISKS, PREFABRICATED, OFF-THE-SHELF |
| L0626 | LUMBAR ORTHOSIS, SAGITTAL CONTROL, WITH RIGID POSTERIOR PANEL(S), POSTERIOR EXTENDS FROM L-1 TO BELOW L-5 VERTEBRA, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, STAYS, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0641 | LUMBAR ORTHOSIS, SAGITTAL CONTROL, WITH RIGID POSTERIOR PANEL(S), POSTERIOR EXTENDS FROM L-1 TO BELOW L-5 VERTEBRA, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, STAYS, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED, OFF-THE-SHELF |
| L0627 | LUMBAR ORTHOSIS, SAGITTAL CONTROL, WITH RIGID ANTERIOR AND POSTERIOR PANELS, POSTERIOR EXTENDS FROM L-1 TO BELOW L-5 VERTEBRA, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES MAY INCLUDE PADDING, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0642 | LUMBAR ORTHOSIS, SAGITTAL CONTROL, WITH RIGID ANTERIOR AND POSTERIOR PANELS, POSTERIOR EXTENDS FROM L-1 TO BELOW L-5 VERTEBRA, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES MAY INCLUDE PADDING, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED, OFF-THE-SHELF |
| L0630 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL CONTROL, WITH RIGID POSTERIOR PANEL(S), POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, STAYS, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0643 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL CONTROL, WITH RIGID POSTERIOR PANEL(S), POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, STAYS, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED, OFF-THE-SHELF |
| L0631 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL CONTROL, WITH RIGID ANTERIOR AND POSTERIOR PANELS, POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0648 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL CONTROL, WITH RIGID ANTERIOR AND POSTERIOR PANELS, POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED, OFF-THE-SHELF |
| L0633 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL-CORONAL CONTROL, WITH RIGID POSTERIOR FRAME/PANEL(S), POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, LATERAL STRENGTH PROVIDED BY RIGID LATERAL FRAME/PANELS, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, STAYS, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0649 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL-CORONAL CONTROL, WITH RIGID POSTERIOR FRAME/PANEL(S), POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, LATERAL STRENGTH PROVIDED BY RIGID LATERAL FRAME/PANELS, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, STAYS, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED, OFF-THE-SHELF |
| L0637 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL-CORONAL CONTROL, WITH RIGID ANTERIOR AND POSTERIOR FRAME/PANELS, POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, LATERAL STRENGTH PROVIDED BY RIGID LATERAL FRAME/PANELS, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0650 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL-CORONAL CONTROL, WITH RIGID ANTERIOR AND POSTERIOR FRAME/PANELS, POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, LATERAL STRENGTH PROVIDED BY RIGID LATERAL FRAME/PANELS, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON INTERVERTEBRAL DISCS, INCLUDES STRAPS, CLOSURES, MAY INCLUDE PADDING, SHOULDER STRAPS, PENDULOUS ABDOMEN DESIGN, PREFABRICATED, OFF-THE-SHELF |
| L0639 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL-CORONAL CONTROL, RIGID SHELL(S)/PANEL(S), POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, ANTERIOR EXTENDS FROM SYMPHYSIS PUBIS TO XYPHOID, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, OVERALL STRENGTH IS PROVIDED BY OVERLAPPING RIGID MATERIAL AND STABILIZING CLOSURES, INCLUDES STRAPS, CLOSURES, MAY INCLUDE SOFT INTERFACE, PENDULOUS ABDOMEN DESIGN, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L0651 | LUMBAR-SACRAL ORTHOSIS, SAGITTAL-CORONAL CONTROL, RIGID SHELL(S)/PANEL(S), POSTERIOR EXTENDS FROM SACROCOCCYGEAL JUNCTION TO T-9 VERTEBRA, ANTERIOR EXTENDS FROM SYMPHYSIS PUBIS TO XYPHOID, PRODUCES INTRACAVITARY PRESSURE TO REDUCE LOAD ON THE INTERVERTEBRAL DISCS, OVERALL STRENGTH IS PROVIDED BY OVERLAPPING RIGID MATERIAL AND STABILIZING CLOSURES, INCLUDES STRAPS, CLOSURES, MAY INCLUDE SOFT INTERFACE, PENDULOUS ABDOMEN DESIGN, PREFABRICATED, OFF-THE-SHELF |
| L1652 | HIP ORTHOSIS, BILATERAL THIGH CUFFS WITH ADJUSTABLE ABDUCTOR SPREADER BAR, ADULT SIZE, PREFABRICATED, INCLUDES FITTING AND ADJUSTMENT, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L1653 | HIP ORTHOSIS, BILATERAL THIGH CUFFS WITH ADJUSTABLE ABDUCTOR SPREADER BAR, ADULT SIZE, PREFABRICATED, OFF THE SHELF |
| L1810 | KNEE ORTHOSIS, ELASTIC WITH JOINTS, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L1812 | KNEE ORTHOSIS, ELASTIC WITH JOINTS, PREFABRICATED, OFF-THE-SHELF |
| L1820 | KNEE ORTHOSIS, ELASTIC WITH CONDYLAR PADS AND JOINTS, WITH OR WITHOUT PATELLAR CONTROL, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L1821 | KNEE ORTHOSIS, ELASTIC WITH CONDYLAR PADS AND JOINTS, WITH OR WITHOUT PATELLAR CONTROL, PREFABRICATED, OFF THE SHELF |
| L1832 | KNEE ORTHOSIS, ADJUSTABLE KNEE JOINTS (UNICENTRIC OR POLYCENTRIC), POSITIONAL ORTHOSIS, RIGID SUPPORT, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L1833 | KNEE ORTHOSIS, ADJUSTABLE KNEE JOINTS (UNICENTRIC OR POLYCENTRIC), POSITIONAL ORTHOSIS, RIGID SUPPORT, PREFABRICATED, OFF-THE SHELF |
| L1843 | KNEE ORTHOSIS, SINGLE UPRIGHT, THIGH AND CALF, WITH ADJUSTABLE FLEXION AND EXTENSION JOINT (UNICENTRIC OR POLYCENTRIC), MEDIAL-LATERAL AND ROTATION CONTROL, WITH OR WITHOUT VARUS/VALGUS ADJUSTMENT, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L1851 | KNEE ORTHOSIS (KO), SINGLE UPRIGHT, THIGH AND CALF, WITH ADJUSTABLE FLEXION AND EXTENSION JOINT (UNICENTRIC OR POLYCENTRIC), MEDIAL-LATERAL AND ROTATION CONTROL, WITH OR WITHOUT VARUS/VALGUS ADJUSTMENT, PREFABRICATED, OFF-THE-SHELF |
| L1845 | KNEE ORTHOSIS, DOUBLE UPRIGHT, THIGH AND CALF, WITH ADJUSTABLE FLEXION AND EXTENSION JOINT (UNICENTRIC OR POLYCENTRIC), MEDIAL-LATERAL AND ROTATION CONTROL, WITH OR WITHOUT VARUS/VALGUS ADJUSTMENT, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L1852 | KNEE ORTHOSIS (KO), DOUBLE UPRIGHT, THIGH AND CALF, WITH ADJUSTABLE FLEXION AND EXTENSION JOINT (UNICENTRIC OR POLYCENTRIC), MEDIAL-LATERAL AND ROTATION CONTROL, WITH OR WITHOUT VARUS/VALGUS ADJUSTMENT, PREFABRICATED, OFF-THE-SHELF |
| L1847 | KNEE ORTHOSIS, DOUBLE UPRIGHT WITH ADJUSTABLE JOINT, WITH INFLATABLE AIR SUPPORT CHAMBER(S), PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L1848 | KNEE ORTHOSIS, DOUBLE UPRIGHT WITH ADJUSTABLE JOINT, WITH INFLATABLE AIR SUPPORT CHAMBER(S), PREFABRICATED, OFF-THE-SHELF |
| L1932 | ANKLE FOOT ORTHOSIS, RIGID ANTERIOR TIBIAL SECTION, TOTAL CARBON FIBER OR EQUAL MATERIAL, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L1933 | ANKLE FOOT ORTHOSIS, RIGID ANTERIOR TIBIAL SECTION, TOTAL CARBON FIBER OR EQUAL MATERIAL, PREFABRICATED, OFF-THE-SHELF |
| L1951 | ANKLE FOOT ORTHOSIS, SPIRAL, (INSTITUTE OF REHABILITATIVE MEDICINE TYPE), PLASTIC OR OTHER MATERIAL, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L1952 | ANKLE FOOT ORTHOSIS, SPIRAL, (INSTITUTE OF REHABILITATIVE MEDICINE TYPE), PLASTIC OR OTHER MATERIAL, PREFABRICATED, OFF-THE-SHELF |
| L3677 | SHOULDER ORTHOSIS, SHOULDER JOINT DESIGN, WITHOUT JOINTS, MAY INCLUDE SOFT INTERFACE, STRAPS, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L3678 | SHOULDER ORTHOSIS, SHOULDER JOINT DESIGN, WITHOUT JOINTS, MAY INCLUDE SOFT INTERFACE, STRAPS, PREFABRICATED, OFF-THE-SHELF |
| L3760 | ELBOW ORTHOSIS (EO), WITH ADJUSTABLE POSITION LOCKING JOINT(S), PREFABRICATED, ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L3761 | ELBOW ORTHOSIS (EO), WITH ADJUSTABLE POSITION LOCKING JOINT(S), PREFABRICATED, OFF-THE-SHELF |
| L3807 | WRIST HAND FINGER ORTHOSIS, WITHOUT JOINT(S), PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L3809 | WRIST HAND FINGER ORTHOSIS, WITHOUT JOINT(S), PREFABRICATED, OFF-THE-SHELF, ANY TYPE |
| L3915 | WRIST HAND ORTHOSIS, INCLUDES ONE OR MORE NONTORSION JOINT(S), ELASTIC BANDS, TURNBUCKLES, MAY INCLUDE SOFT INTERFACE, STRAPS, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L3916 | WRIST HAND ORTHOSIS, INCLUDES ONE OR MORE NONTORSION JOINT(S), ELASTIC BANDS, TURNBUCKLES, MAY INCLUDE SOFT INTERFACE, STRAPS, PREFABRICATED, OFF-THE-SHELF |
| L3917 | HAND ORTHOSIS, METACARPAL FRACTURE ORTHOSIS, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L3918 | HAND ORTHOSIS, METACARPAL FRACTURE ORTHOSIS, PREFABRICATED, OFF-THE-SHELF |
| L3923 | HAND FINGER ORTHOSIS, WITHOUT JOINTS, MAY INCLUDE SOFT INTERFACE, STRAPS, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L3924 | HAND FINGER ORTHOSIS, WITHOUT JOINTS, MAY INCLUDE SOFT INTERFACE, STRAPS, PREFABRICATED, OFF-THE-SHELF |
| L3929 | HAND FINGER ORTHOSIS, INCLUDES ONE OR MORE NONTORSION JOINT(S), TURNBUCKLES, ELASTIC BANDS/SPRINGS, MAY INCLUDE SOFT INTERFACE MATERIAL, STRAPS, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L3930 | HAND FINGER ORTHOSIS, INCLUDES ONE OR MORE NONTORSION JOINT(S), TURNBUCKLES, ELASTIC BANDS/SPRINGS, MAY INCLUDE SOFT INTERFACE MATERIAL, STRAPS, PREFABRICATED, OFF-THE-SHELF |
| L4360 | WALKING BOOT, PNEUMATIC AND/OR VACUUM, WITH OR WITHOUT JOINTS, WITH OR WITHOUT INTERFACE MATERIAL, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L4361 | WALKING BOOT, PNEUMATIC AND/OR VACUUM, WITH OR WITHOUT JOINTS, WITH OR WITHOUT INTERFACE MATERIAL, PREFABRICATED, OFF-THE-SHELF |
| L4386 | WALKING BOOT, NON-PNEUMATIC, WITH OR WITHOUT JOINTS, WITH OR WITHOUT INTERFACE MATERIAL, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L4387 | WALKING BOOT, NON-PNEUMATIC, WITH OR WITHOUT JOINTS, WITH OR WITHOUT INTERFACE MATERIAL, PREFABRICATED, OFF-THE-SHELF |
| L4396 | STATIC OR DYNAMIC ANKLE FOOT ORTHOSIS, INCLUDING SOFT INTERFACE MATERIAL, ADJUSTABLE FOR FIT, FOR POSITIONING, MAY BE USED FOR MINIMAL AMBULATION, PREFABRICATED ITEM THAT HAS BEEN TRIMMED, BENT, MOLDED, ASSEMBLED, OR OTHERWISE CUSTOMIZED TO FIT A SPECIFIC PATIENT BY AN INDIVIDUAL WITH EXPERTISE | L4397 | STATIC OR DYNAMIC ANKLE FOOT ORTHOSIS, INCLUDING SOFT INTERFACE MATERIAL, ADJUSTABLE FOR FIT, FOR POSITIONING, MAY BE USED FOR MINIMAL AMBULATION, PREFABRICATED, OFF-THE-SHELF |
Publication History
| March 27, 2014 | Originally Published |
| April 30, 2015 | Revised to add 2015 HCPCS table |
| July 2, 2015 | Revised typographical errors in HCPCS codes narrative descriptions for L0627 and L0642 |
| March 28, 2019 | Clarified custom fit requirements |
| July 05, 2019 | Updated HCPCS codes K0901 and K0902 to L1851and L1852 respectively |
| March 11, 2021 | Revised to remove HCPCS codes with no OTS equivalent and to add HCPCS codes L3760 and L3761. Please refer to the bulletin article "Custom Fitted Orthotic HCPCS Codes Without a Corresponding Off-the-Shelf Code – Correct Coding" for additional information |
| February 1, 2024 | Revised to remove "Use of CAD/CAM or similar technology to create an orthosis without a positive model of the patient may be considered as OTS if the final fitting upon delivery to the patient requires minimal self-adjustment as described in this section." and "Use of CAD/CAM or similar technology to create an orthosis without a positive model of the patient may be considered as custom fitted if the final fitting upon delivery to the patient requires more than minimal self-adjustment requiring expertise as described in this section." and to add "As a reminder, use of CAD/CAM or additive manufacturing techniques alone does not designate a product as custom fabricated." |
| October 10, 2024 | Revised to add L1652 and L1653 as a corresponding code set, and to add L1820 and L1821 as a corresponding code set |
| April 3, 2025 | Revised to add L1951 and L1952 as a corresponding code set, and to add L1932 and L1933 as a corresponding code set |

