Status Report for Quarter 3 2013: HCPCS Code E0601 — Service-Specific Prepayment Review
The Medical Review Department of CGS, the Jurisdiction C DME MAC, continues its service-specific prepayment edit for HCPCS code E0601 (Continuous Airway Pressure [CPAP] Device). This edit is the result of data demonstrating a high claims payment error rate for this product category.
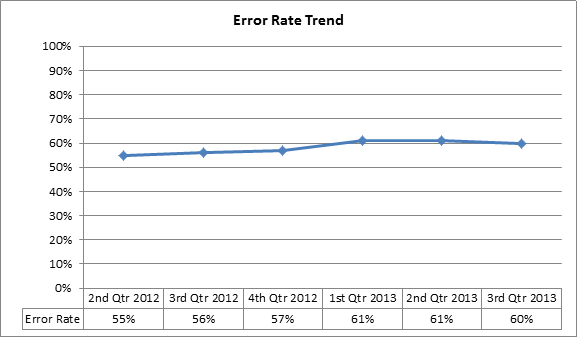
The claim review for July 1, 2013 – September 30, 2013 was only for the initial month of therapy (E0601RRKH). A summary report follows:
| Current
Quarter E0601RRKH |
Previous
Quarter E0601RRKH |
|
|---|---|---|
| Denial Rate | 56% | 67% |
| Allowed Dollars Error Rate | 60% | 61% |
Non-response Rate to Additional Documentation Requests – 9%
An analysis of the claim denials showed that the top 10 reasons a determination was made not to pay the claim were:
- The documentation did not include a copy of a board certification document, screen print from a national certification agency, etc. that verifies that the physician who interpreted the sleep test met policy requirements.
- The medical records provided did not include documentation of a face-to-face clinical evaluation, conducted by the treating physician prior to the sleep study, which assessed the beneficiary for OSA.
- A copy of the sleep test interpretation was not provided.
- The sleep study was not interpreted by a physician with credentials meeting policy requirements or the proof of credentialing that was provided was not obtained from the website of a national certification agency or state medical board.
- The author's signature was missing or illegible on a key piece of medical documentation, so the information could not be authenticated.
- The supplier's records did not document that the beneficiary and/or their caregiver received instruction in the proper care of the PAP device and accessories.
- The claim was for a replacement PAP device due to the previous equipment reaching its reasonable useful lifetime (RUL) and the documentation either did not include a recent face-to-face evaluation or the evaluation did not confirm that the beneficiary continued to use and benefit from the PAP device.
- The beneficiary began using PAP prior to Medicare eligibility and the documentation did not include a F2F evaluation conducted following enrollment in FFS Medicare or the note did not record that the beneficiary had a diagnosis of OSA and continued to use and benefit from PAP therapy.
- Proof of delivery was not provided or was incomplete.
- The item(s) was delivered before the supplier obtained a detailed written order and the records that were provided did not include written documentation of a preliminary dispensing order.
Case Study
A.A: 60 year old female
DOS: 08/28/2013
Items on claim
| E0601 | Continuous Airway Pressure (CPAP) Device |
| E0562 | Humidifier, Heated, Used With Positive Airway Pressure Device |
| A7030 | Full Face Mask Used With Positive Airway Pressure Device |
| A7035 | Headgear Used With Positive Airway Pressure Device |
| A7037 | Tubing Used With Positive Airway Pressure Device |
| A7038 | Filter, Disposable, Used With Positive Airway Pressure Device |
| A7039 | Filter, Non Disposable, Used With Positive Airway Pressure Device |
| A7046 | Water Chamber For Humidifier, Used With Positive Airway Pressure Device, Replacement |
Delivery Documentation: Included the name and address of the beneficiary and a detailed list of items that corresponded with the HCPCS codes submitted on the claim. The beneficiary signed the delivery slip and personally dated her signature. The signature date was 08/28/2013.
Detailed Written Order: Signed by the ordering physician on 08/19/2013. All items billed on the claim were also listed on the DWO. The quantity to dispense was "1" for every item except the disposable filters (A7038). The quantity to dispense for that item was "6". The length of need was listed as 99 (lifetime).
Dispensing Order: N/A – DWO was obtained prior to delivery.
Face-to-Face Exam: Dated May 7, 2013
Height: 62 inches – Weight: 246 pounds – BMI: 45.0
Beneficiary was unable to sleep at night and admitted to snoring in her sleep. Her husband related that she sometimes quit breathing in her sleep. She felt tired and sleepy during the day. She has a history of hypertension. No history of diabetes or heart disease. "Probable OSA given by her clinical history and body habitus" was listed as one of the diagnoses and the physician's plan of care included a polysomnogram.
Diagnostic Study: Overnight facility-based PSG on 07/12/2013.
Total recording time was 391.5 minutes with a total sleep time of 303.5 minutes. Sleep efficiency was 77.5%. Study showed 17 obstructive apneas, 0 central and 0 mixed apneas and 232 hypoxic events noted. Overall AHI was 49.2.
Titration Study: 08/11/2013 – CPAP therapy recommended at 13 cm of H2O with warm humidifier
Credentials for Interpreting Physician: Printout from Georgia Medical Board that showed a specialty in Neurology with a subspecialty in sleep medicine from the American Board of Psychiatry and Neurology (ABPN).
Beneficiary Education: The signed delivery slip included the following statement: "I have received proper documentation and instruction in how to properly clean and maintain my CPAP or BIPAP machine and cleaning instructions for my mask (Model) 61700.
Medical Review Decision: Paid all codes except A7046 (replacement for humidifier water chamber). The fee schedule for humidifiers used in conjunction with PAP therapy includes the initial water chamber. HCPCS code A7046 should only be used to bill for a replacement. Since suppliers may only bill for 90 days of supplies at a time and payment for a replacement water chamber is only available every 6 months, HCPCS code A7046 should not be submitted on the initial claim for PAP therapy.
Additionally, water chambers are non-consumable supplies so, even if Medicare allows payment for a replacement every six months, suppliers should not automatically bill for a new one according to that time interval. Suppliers are expected to assess whether supplies remain functional and only provide a replacement when the item is no longer able to be used safely and effectively for the purpose for which it was intended. Suppliers are required to document the functional condition of the item(s) being replaced in sufficient detail to demonstrate the cause of the dysfunction that necessitates replacement.
For additional information related to the refill/replacement requirements for non-consumable supplies, refer to the online article, "Refill Requirements for Non-consumable Supplies – Frequently Asked Questions."

